
Translations for all marketing authorisation procedures for medicinal products that reliably comply with the regulations
Professional in all 24 EU languages and 26 EEA languages
The marketing authorisation for medicinal products is strictly regulated in Europe and usually requires extensive technical translations of various medical and pharmaceutical documents. As a specialised language service provider, mpü has developed excellent solutions for all medicinal products that are both tailored to international authorisations and adapted to national regulations.
- All medicinal products
including all special areas such as:- Paediatric medicinal products
- Oncological medicinal products
- Orphan drugs
- Veterinary medicinal products
- etc.
- Generic drugs
- Biosimilars
We speak 150 languages
Regulatory labelling – linguistic expertise in all phases of the life cycle
mpü offers you a full package of solutions and services for the authorisation of medicinal products in strict compliance with all regulations issued by the European Medicines Agency (EMA) and other country-specific authorities.
- Creation of the English product information (SmPC, PIL, labelling), also known as:
Summary of product characteristics (SmPC) | Patient information leaflet (PIL) | Labelling, labels, text on inner and outer packaging - Translation into 26 QC-tested language versions
- Quality assurance
- Readability user test
- Linguistic review
Translations for marketing authorisation procedures: planning ahead – flexible time management
mpü processes assure far-sighted planning of the entire translation process and timely delivery of correct and reviewed documents in the national languages to ensure compliance with the submission deadlines.
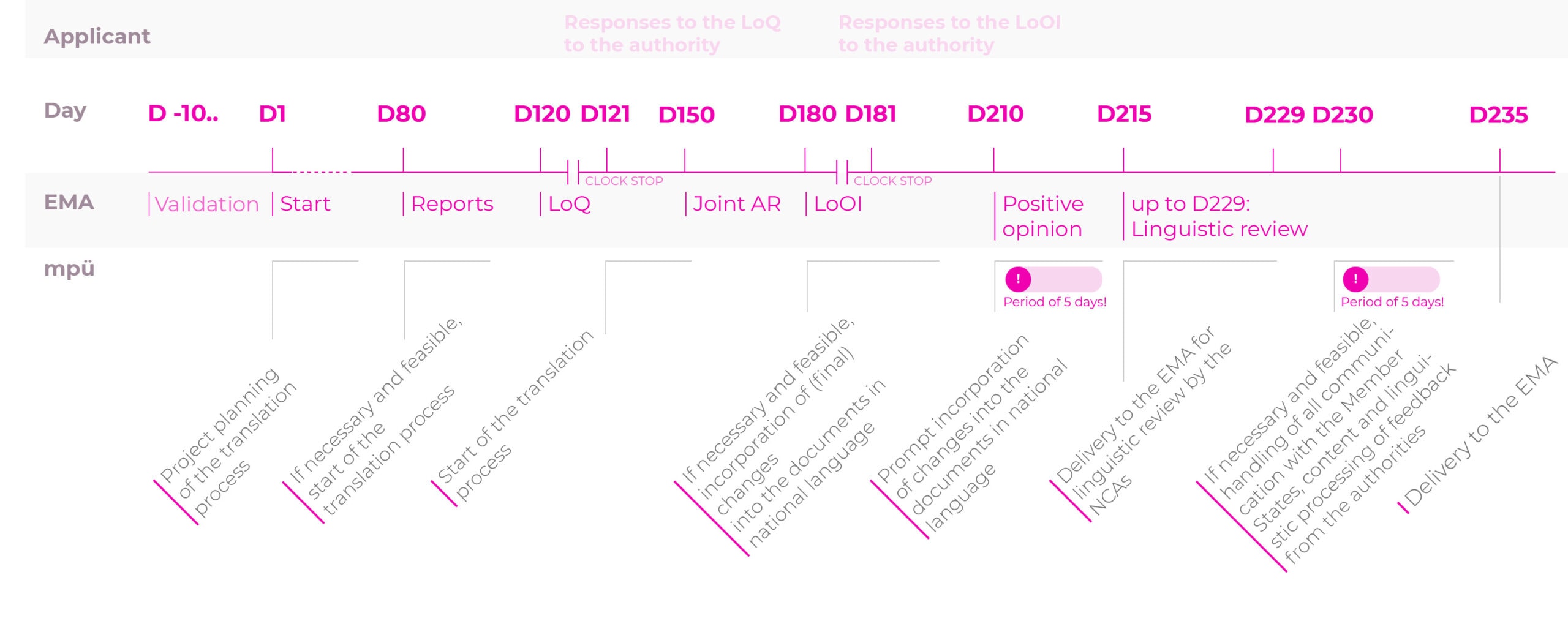
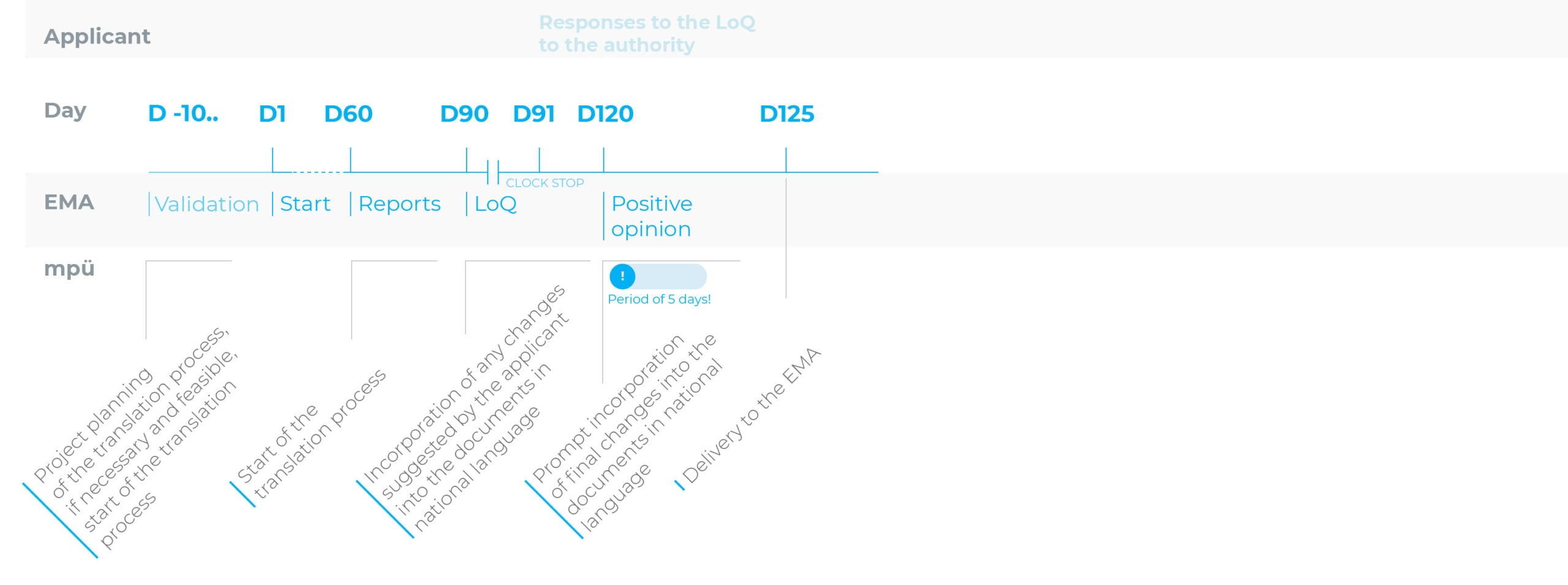
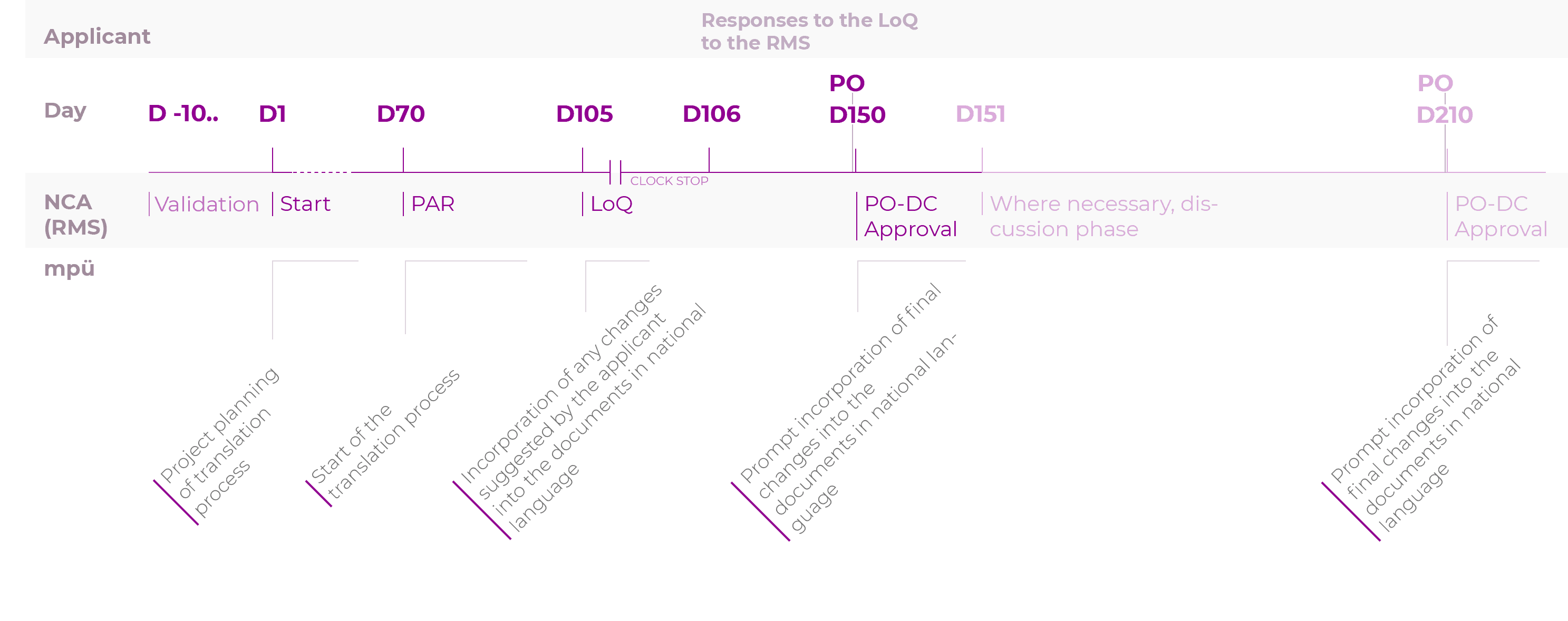
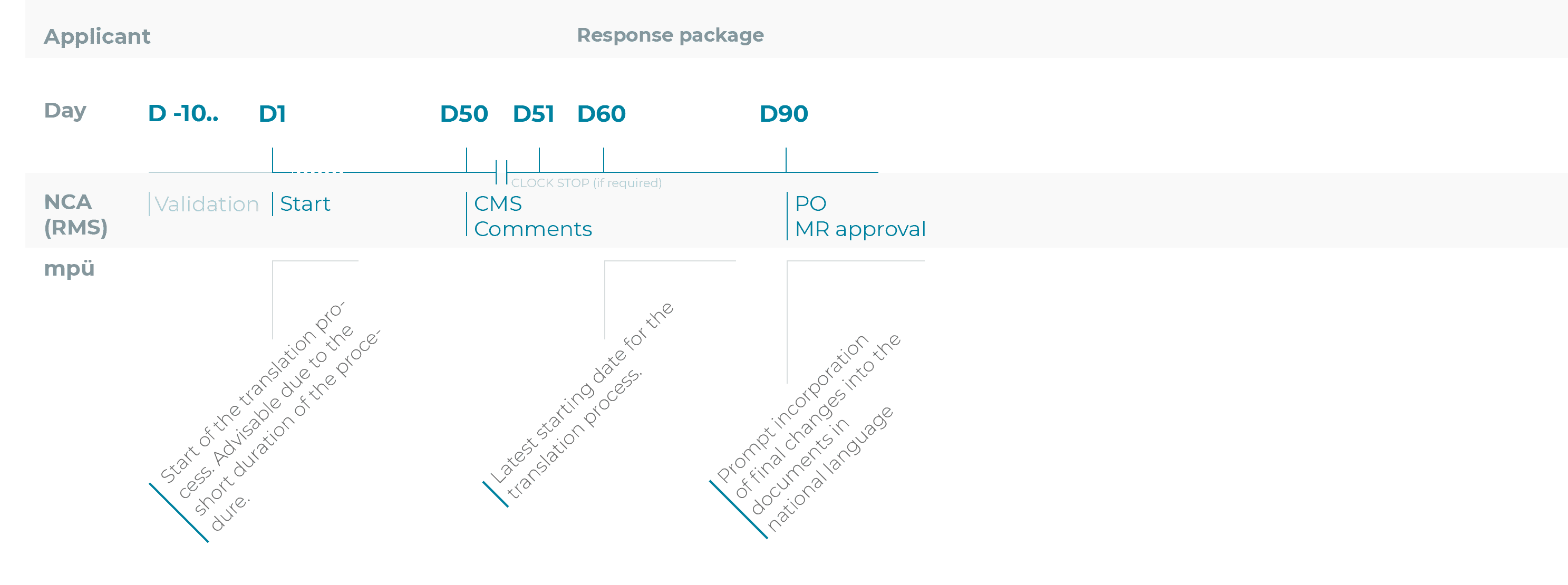
Timelines for the translation process for the registration procedures
Centralised procedure (CP): normal procedure
Centralised procedure (CP): accelerated procedure
Decentralised authorisation procedure (DCP)
Mutual recognition procedure (MRP)
Always available for you quickly at all times
Compliance with short registration deadlines
To make sure you do not miss the deadline for submitting all documents in the national languages after receiving a positive opinion (CP) or after the approval (MRP/DCP), we recommend starting the translation process of the source texts or the transfer of the revised parts of the text into the documents in national language as early as possible.
In other words, this process should ideally be started as early as the day of submission of the application for marketing authorisation or variation/the variation notification.
The 5-day period after a positive opinion (CP) or after approval (DCP/MRP) should be reserved for making last-minute changes only.
Weekend work: no problem at mpü.
With the centralised procedure (CP), we will work for you even over the weekend for the 5-day period after the positive opinion so we can finalise all of the language versions promptly.
Adaptations to reflect the current QRD template versions and reviews/proofreading of existing translations can be completed prior to the completion of the application assessment or before receipt of the positive opinion (CP) or approval (DCP/MRP) in preparation for the next step.
Our fast response time ensures your deadline will be met
Translations of all product information
All product information is usually written in English and subsequently translated into all relevant EU languages. It includes:
SmPC, PIL and labelling, also known as
- Summary of product characteristics (SPC or SmPC)
- Patient information leaflet (PIL) or package leaflet (PL)
- Labels (text on inner and outer packaging)
Life cycle management
Handling of the linguistic review with Member States
- Established contacts with partners in the authorities of all EU Member States
- Handling of all of the communication between authorities and translators
- Content and linguistic processing of feedback from the authorities
- Rapid problem solving based on medical and regulatory expertise in all queries and finalisation of the labelling (QRD Form 2)
We are renowned for producing the highest quality for over 45 years.
Registration dossiers
Preparation and translation of all 5 modules of the common technical document (CTD)
The regulations for regulatory approval of medicinal products are largely harmonised in Europe. The common technical document (CTD or eCTD) serves as the basis for the marketing authorisation of medicinal products on the European market.
In addition to administrative information, it contains detailed information on the pharmaceutical quality and manufacture, efficacy, and safety of the medicinal product (verified by documentation of clinical and non-clinical studies).
Our process includes all steps for a smooth submission both of the entire dossier and the individual documents or modules.









