
Translations for clinical trials/clinical studies
Regulatory challenge and patient safety for translations of clinical trials
Six of the top ten companies in the pharmaceutical industry rely on mpü’s expertise for translations of clinical trials/clinical studies.
Clinical trials are increasingly conducted across national and language borders in very different countries. This internationality and therefore the diversity of languages spoken by subjects as well as any special features resulting from the geographical location are an enormous challenge faced by companies when carrying out and evaluating clinical studies.
We take on your challenges and support you through all of the stages of your medicinal product development and clinical trials.
Quality assurance in the translation of documents from clinical trials
mpü quality assurance – skilled and precise
mpü has many years of experience of all stages of medicinal product development and marketing authorisation. With our experienced translation and language management team, we meet all of the requirements of multilingual clinical studies.
According to the regulations and recommendations of the
- EMA/FDA*/ICH-GCP guidelines and
- the authorities and ethics committees.
The priority of the internal mpü quality assurance is to ensure that all of the documents and information are created in a reliable and precise way for the respective target group and target country in the mother tongue of the study participants and localised in an understandable way.
Only perfect and localised translations ensure that patients both a willingness to participate on the part of the patients and their safety.
mpü quality assurance – the steps to getting a high-quality product
1
mpü is certified according to DIN EN ISO 17100 and your translations will be carried out according to the process for this internationally applicable quality standard.
However, our multi-stage quality assurance workflow based on DIN EN ISO 17100 is supplemented by additional mpü-specific steps such as in-house quality assurance by qualified linguists.
2
Regional and local conventions and standards are taken into account by the native speaker translators and reviewers.
3
Back translation and revision and a comparison of the translation for documents from clinical trials such as patient information, declarations of consent and QoL, QuIC and PRO questionnaires.
4
An experienced team of expert project and quality managers will support you in a flexible way throughout all of the steps in your project. Our target-oriented customer communication always keeps you up to date.
5
Qualification of the native technical translators and reviewers for clinical trials.
6
Foreign language DTP and manner correction of the documents used by native editors.
7
Identification and maintenance of terminology
Ongoing improvement to text quality using terminology management.
Company-wide and cross-department adherence to the specialist terminology corporate language is ensured.
8
Translation memory
Translation projects are carried out using translation memory systems, which are translation tools integrated into the workflow which help save costs, reduce turnaround times, ensure consistency across texts and maintain formatting.
Quality doesn’t happen by chance
mpü process –
linguistic validation and cognitive debriefing of PRO under one roof
Since clinical trials/clinical studies are increasingly being conducted at an international level, there is also a growing need for translation, harmonisation and cultural adaptation of patient-reported outcome measures (PROM) and for linguistic validation of the PROMs.
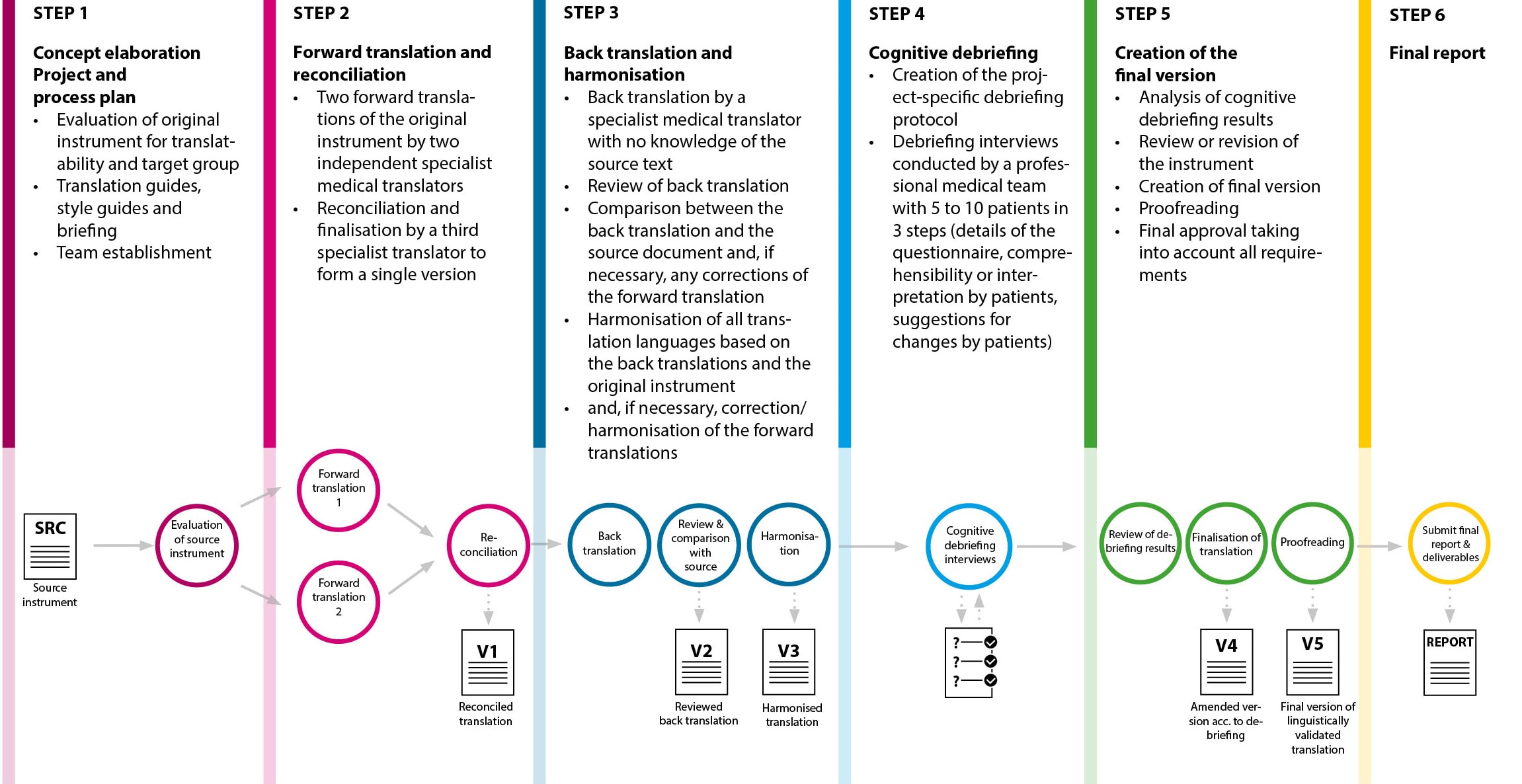
For PROMs, you can select a complete package for linguistic validation including cognitive debriefing or just select individual steps.
mpü – your expert for linguistic validation with cognitive debriefing of PROM
Translations + patient recruitment + testing under one roof
- Process BEFORE the start of the study to ensure that the study participants understand the content of the questionnaires properly
- Translations, where appropriate with back translations, of all questionnaires that will need to be completed by the study participants during the study
- Translations and cultural adaptation in all languages of the study countries
- Patient recruitment in all languages of the study countries
- Implementation of the tests (cognitive debriefing) in the study countries
- Patients interpret and evaluate the study questionnaires translated into their mother tongue to determine how easy they are to understand with no interference by the interviewer
- Depending on the result, the source text or the translation into individual languages may potentially need to be revised
- Process BEFORE the start of the study to ensure that the study participants understand the content of the questionnaires properly
- Translations, where appropriate with back translations, of all questionnaires that will need to be completed by the study participants during the study
- Translations and cultural adaptation in all languages of the study countries
- Patient recruitment in all languages of the study countries
- Implementation of the tests (cognitive debriefing) in the study countries
- Patients interpret and evaluate the study questionnaires translated into their mother tongue to determine how easy they are to understand with no interference by the interviewer
- Depending on the result, the source text or the translation into individual languages may potentially need to be revised
Translations must be understood by everyone
mpü expertise
- mpü will support you with the development of strategies from the first steps of linguistic validation including the cognitive debriefing of PROMs.
- mpü will provide reliable PRO instruments which enable the successful implementation of your global studies.
- The mpü processes guarantee official acceptance.
- mpü will support you with the development of strategies from the first steps of linguistic validation including the cognitive debriefing of PROMs.
- mpü will provide reliable PRO instruments which enable the successful implementation of your global studies.
- The mpü processes guarantee official acceptance.
We live and breathe medical translations
Our network – your success
The global mpü network of
- Medical translators
- Subject experts
- Doctors
- In-country reviewers and interviewers
- Linguistic validators
means we have the option to carry out both the patient recruitment and the cognitive debriefing in the target country. Through this mpü procedure, we can ensure the scientific accuracy of the PROMs
The cognitive debriefing of the translated document is a step that is critical to the results. This process allows us to ensure that all patients understand the PROMs correctly and in the same way and are able to complete them in all target languages at a subsequent stage in the study.
From a linguistic perspective, each mpü project is equivalent in all languages. You can rely on your data from all countries being able to be pooled for the statistical analyses.
The quality standard for linguistic validation processes and the cognitive debriefing of PRO
The mpü expertise in the field of the translation and harmonisation of paper-based or electronic PRO instruments is based on our focus on translations of documents for globally active pharmaceutical companies and CROs.
The mpü processes and quality standard for the linguistic validation and the cognitive debriefing of PROMs follow all important guidelines:
- PRO guideline of the American Food and Drug Administration (FDA)
- Regulations of the European Medicines Agency (EMA)
- ICH-GCP standards
- Guidelines of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR).
All stages of marketing authorisation for medicinal products
With its goal of ‘100% quality’, mpü sets the most stringent standards for adapting all processes to the individual documents and implements them at every phase of medicinal product development:
- Preclinical
- Clinical phases I – IV
- Post-marketing surveillance studies
- Non-interventional studies (NIS)
Thanks to our specialised translation and language solutions that take into account a wide range of needs and working conditions, we successfully support globally active companies and CROs.
All medical specialties in 150 languages
You can take advantage of the full range of mpü services in all medical specialties as our linguistic and subject-matter experts have a great deal of experience in their subject areas.
These include, among others:
- Gynaecology
- Geriatrics
- Cardiology
- Dentistry
- Neurology
- Oncology
- Orthopaedics
- etc.
Different types of documents for
patients – investigators – authorities
require different processes
patients – investigators – authorities
require different processes
Because the different types of documents are intended for different target groups (patients, investigators, authorities, ethics committees), the translations require different processes appropriate for the target group and the subject matter of the document:
- Informed consent forms (ICFs)
- Patient information (PISs)
- Case report forms (CRFs, eCRFs)
- Patient diaries and e-diaries
mehr
- Patient cards
- Patient questionnaires
- Patient-reported outcomes (PRO)
- Investigator’s brochures
- Protocol synopses
- Medical records
- AE/SAE/SUSAR – reports (phamacovigilance)
- Protocols
- Monitoring and audit reports
- Study reports
- Doctors’ letters
- Trial sites – documentation and reports
- Safety reports
- Packaging and labelling of the investigational medicinal product, label check
- Patient information leaflets, package inserts (PLs/PILs)
- Communication with ethics committees and authorities
- Final reports (clinical trial reports, CTRs)
- Summary of product characteristics (SPC/SmPC)
- Summaries for laypersons
- Contracts
- Confidentiality agreements
- Documents on the training of trial site staff
- Import and export approvals
Special 24-/48-hour service for translations of
adverse event (AE), serious adverse event (SAE) and suspected unexpected serious adverse reaction (SUSAR) reports
mpü has set up a special service to ensure that the time-sensitive reporting of AEs/SAEs/SUSARs can be complied with (24/48 hours).
Our established and tested processes are oriented towards promptly preparing multilingual AE/SAE/SUSAR reports.
The global position of our company and our experts means that we can make the most of different time zones so that this time-critical service can be offered.
Routine – reliable – technically sound
mpü is ISO certified
mpü is certified according to EN DIN ISO 9001 for a smooth process and reliable quality assurance and according to DIN EN ISO 17100 to ensure the quality that your exacting documentation requires.
Certified study documents
mpü delivers a translation certificate for every translation option which contains details of the language and subject experts involved, a precise description of the selected process and the title of the document and language directions.
Attestations, apostilles, etc. can also be provided for every document if required.
Use the mpü foreign language set for the perceft documents
Complete your study documents with our skill in multilingual DTP.
- We adapt translations,
- take into account the linguistic expansion of the various target languages,
- our native speakers review the documents used and
- you receive your documents in your desired final format.
Country-specific label check for translations of labels for your investigational medicinal products
Translation and validation of labels for investigational medicinal products (study drugs) taking into account the standard terminology
+
Label check for the investigational medicinal product labels for each country where study sites are located
Optimal use of resources for the highest quality
When translating labels for investigational medicinal products for clinical trials, the time and effort needed for the translation itself is relatively small. However, what is known as a label check has to be performed for every language, and its scope can vary depending on the country-specific regulations for each language or country.
Thanks to their comprehensive know-how for all language processes related to the marketing authorisation process and clinical trials, mpü has built a wide network of specialists for this specialised service.
We have the ability to respond quickly to your specific requirements and we can develop these customised workflows with all of the steps for you. So your trial can start on time.
Label checks
The country-specific label check for the investigational medicinal product checks and adapts the translation of the master label according to the following criteria:
- Exact content of the master label
- Composition of the preparation
- Investigated therapeutic indication
- Synopsis of the clinical trial (which determines the preparation type and usage conditions)
- Language and country of the study sites to ensure the correct adaptation of the label content and thus compliance with the regulations in force in the respective country or region.
Thanks to our specialised translation and language solutions, we successfully support globally active companies and CROs to meet all their needs related to clinical trials.
Why mpü?
- 1
Our flexibility and expertise enable all globally active companies and CROs to make the most of our capacities as a fully outsourced service or as a partial service provider:
- 2
Expert support from your project manager.
- 3
150 languages in all language combinations.
- 4
Customer-specific technical solutions and workflow design to combine clinical processes with language processes.
- 5
Rapid processing times thanks to optimised workflows and defined practices in process control – precise results.
- 6
Seamless integration with the requirements of the CROs.
- 7
Support for the CROs with tenders – volume discounts and reduced prices for large-scale translation projects and tenders.









