Biotechnology
Biopharmaceuticals and biosimilars –
the growing business areas of the pharmaceutical and biotechnology industry
Medical biotechnology (also known as “red biotechnology”) focuses on the development of new therapeutic and diagnostic procedures. With their innovative approaches and methods of treatment and diagnosis, biopharmaceuticals and biosimilars make valuable new contributions to the life-saving treatment of various clinical pictures.
Almost one in two new products registered in the pharmaceutical industry is manufactured using biotechnology.
Biopharmaceuticals
Professional translations are a requirement for both the studies being carried out at an international level and the current research results and technological progress being able to be exchanged and expanded.
Development, study and registration documentation
According to the EU and FDA requirements and the ICH-GCP standards, the demanding registration procedures for biopharmaceuticals require extensive testing in many countries and in many languages.
The mpü experts are ideally equipped and experienced in translating all of your biopharmaceutical documents on the topics
- Properties (physical, chemical)
- Composition (pharmaceutical, biological)
- Efficacy (PK, PD)
- Tolerability (including immunogenicity)
- Security
- Manufacture
- Registration
- Sales, marketing
- Risk management
- Post-marketing surveillance (pharmacovigilance)
mpü offers specific solutions for new problems
Internationally accessible research results
In biotechnology, findings from the most varied of scientific areas are used. To achieve optimal language solutions, the technical translators and experts at mpü bring with them expertise and experience in all of the necessary subject areas.
As a result, your developments of newer and more efficient procedures in indications such as
- Medical diagnostics
- Pharmacogenomics
- Prenatal diagnostics
- Gene therapy
- Regeneration medicine
- etc.
can be made globally accessible by mpü in all necessary languages.
Biosimilars
Since simple evidence of bioequivalence cannot be provided for biological preparations, the registration of biosimilars is primarily based on comparability studies.
Comparison of data requirements for approval of a biosimilar versus the reference medicine
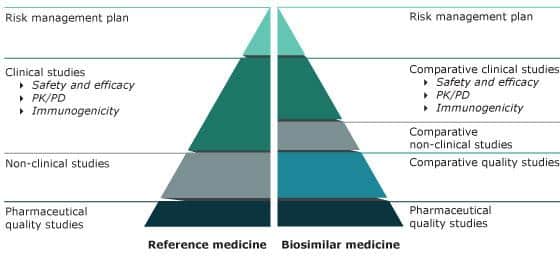
© Grafik: European Medicine Agency (EMA),
https://www.ema.europa.eu/en/human-regulatory/overview/biosimilar-medicines-overview, abgerufen 13JUN2022
The mpü team has the necessary special skills and experience needed for translations from these special procedures into the highly specific subject areas.









