
mpü process –
linguistic validation and cognitive debriefing of PRO
Since clinical trials/clinical studies are increasingly being conducted at an international level, there is also a growing need for translation, harmonisation and cultural adaptation of patient-reported outcome measures (PROM) and for linguistic validation of the PROMs.
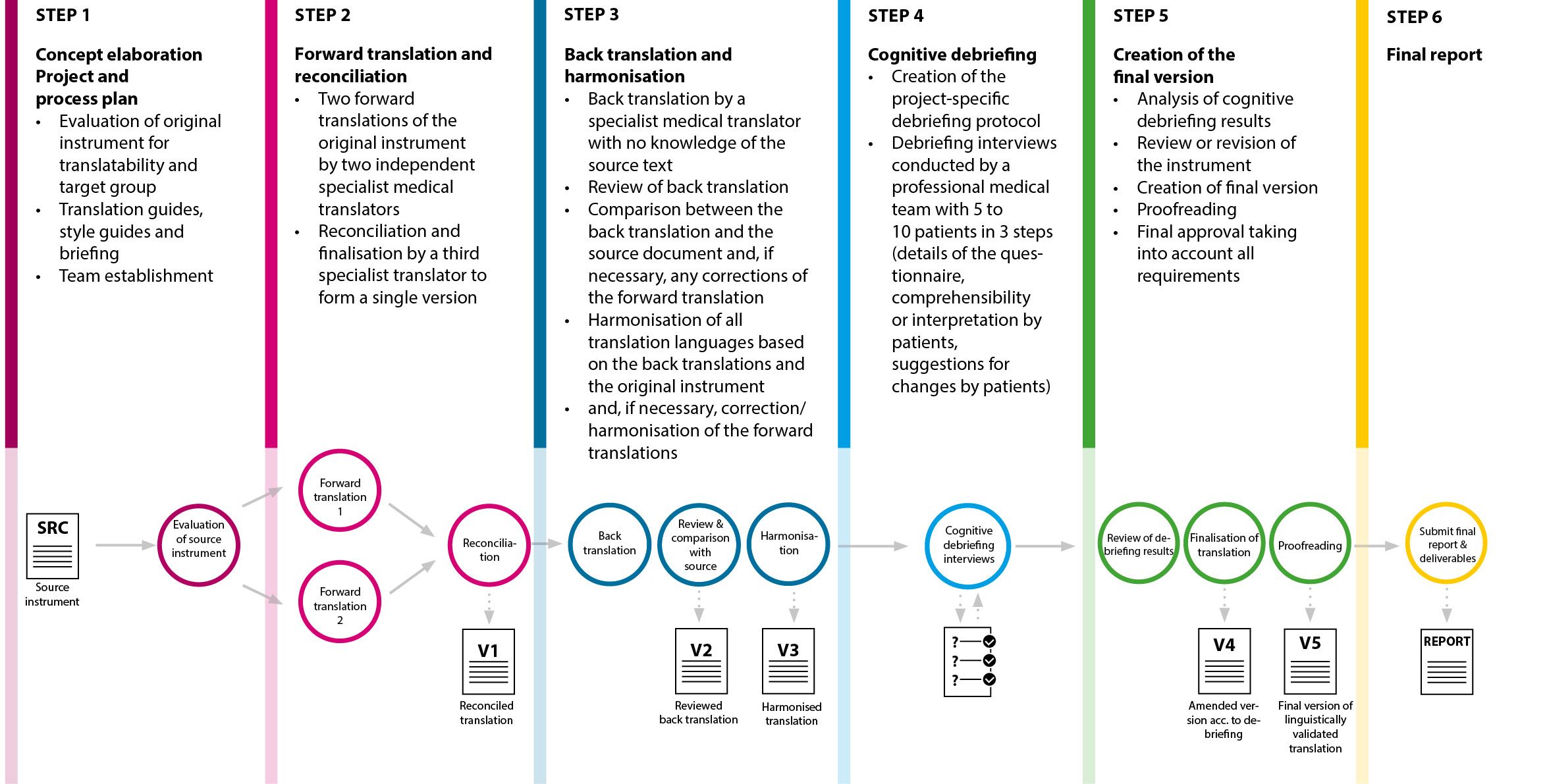
For PROMs, you can select a complete package for linguistic validation including cognitive debriefing or just select individual steps.
mpü – your expert for linguistic validation with cognitive debriefing of PROM
Translations + patient recruitment + testing under one roof
Translations must be understood by everyone
mpü expertise
Our network – your success
The global mpü network of
means we have the option to carry out both the patient recruitment and the cognitive debriefing in the target country. Through this mpü procedure, we can ensure the scientific accuracy of the PROMs.
The cognitive debriefing of the translated document is a step that is critical to the results. This process allows us to ensure that all patients understand the PROMs correctly and in the same way and are able to complete them in all target languages at a subsequent stage in the study.
From a linguistic perspective, each mpü project is equivalent in all languages. You can rely on your data from all countries being able to be pooled for the statistical analyses.
The quality standard for linguistic validation processes and the cognitive debriefing of PRO
The mpü expertise in the field of the translation and harmonisation of paper-based or electronic PRO instruments is based on our focus on translations of documents for globally active pharmaceutical companies and CROs.
The mpü processes and quality standard for the linguistic validation and the cognitive debriefing of PROMs follow all important guidelines:









