
Tailored medical and pharmaceutical translations
For the highly regulated pharmaceutical companies, the factors of
time, cost, quality and time-to-market
are always in focus, including in terms of global markets and translation projects.
mpü doesn’t just provide translations at various levels of quality with reliable and consistent language management. We also offer tailored solutions for our customers.
mpü expertise and philosophy
mpü expertise
Only selected resources and experts – for 45 years this has been our requirement for the successful implementation of a translation project.
In addition to linguistic skill and unconditional reliability, the basis for collaborating on mpü projects is multiple years of experience in the areas of medicine, pharmaceuticals and science.
3,000 native-speaker linguists who have degrees and specialist training and subject matter experts are selected and tested by mpü around the world.
mpü philosophy
It is our philosophy at mpü that practical specialist knowledge of experienced scientists such as doctors, pharmacists, microbiologists, engineers, etc. complements the translation process from a technical perspective. We are able to cover the following subject areas in a qualified way:
- Medicine
- Dentistry
- Veterinary medicine
- Pharmacology and toxicology
- Pharmacy
- Laboratory medicine
- Biochemistry
more
- Biology
- Molecular biology
- Microbiology
- Genetics
- Bioinformatics
- (Nano)biotechnology
- Chemistry
- Physics
- Information technology
- Engineering
- etc.
and the numerous sub-disciplines.
Translation expertise in all phases of the life cycle of medicinal products
The extensive mpü services guide and support you in all stages of the life cycle of a medicinal product or product from research through to marketing:
- Preclinical and clinical research and development
- Clinical studies
- Registration procedures
- Linguistic review
- Manufacturing, labelling and packaging
- Market launch and sales
- Pharmacovigilance (post-marketing surveillance)
For all documents in the area of pharmaceuticals including all modules in the registration dossier (CTD/eCTD), the mpü processes offer all of the steps needed for easy processing through to submission, both for individual documents and for complete dossiers.
mpü helps you to hit the market quickly with your preparations
Reliable translations for all registration procedures.
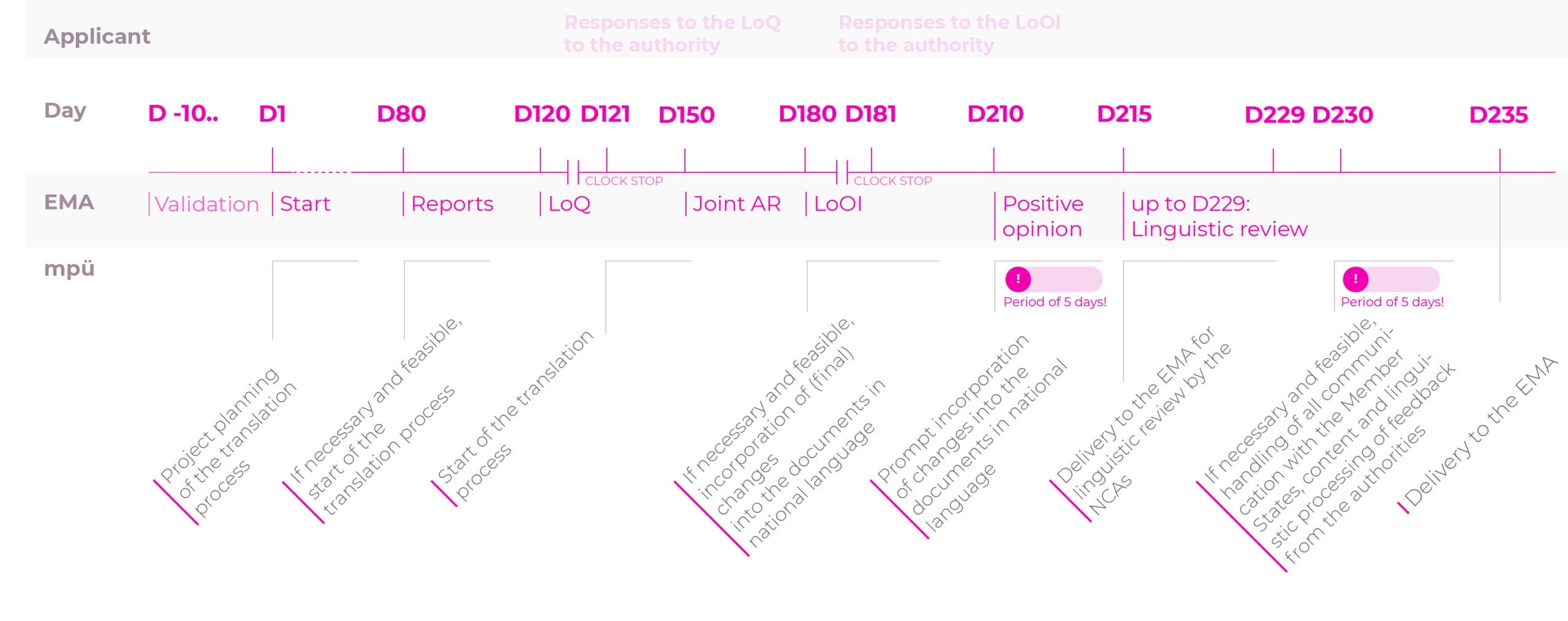
The indicative planning and the prompt delivery of correct and checked documents in the national language makes mpü your reliable partner for all of your registration procedures.
We have shown you the timelines for the mpü translation process for the various registration procedures to make the prompt planning of your projects transparent.
Timelines for the translation process for the registration procedures
Centralised procedure (CP): normal procedure
LIFE CYCLE MANAGEMENT OF YOUR PRODUCT INFORMATION IN 24/26 LANGUAGES
mpü is specialised in the management and coordination of the entire regulatory process for your labelling in all registration procedures according to national and European law.
CP – DCP – MRP – NP – variations – national notifications of amendments (PRAC) – line extensions – renewals – PSURs – PBRER
Our Regulatory Labelling team would be happy to advise you on the best approach to your project.
QUALITY ASSURANCE AND LINGUISTIC VALIDATION IN CLINICAL STUDIES
Patient safety is critical in all translations
According to the regulations and recommendations of the
- EMA/FDA*/ICH-GCP guidelines and the
- authorities and ethics committees.
The priority of the internal mpü quality assurance is to ensure that all of the documents and information are created in a reliable and precise way for the respective target group and target country in the mother tongue of the study participants and localised in an understandable way.
With mpü, your confidential documents are in the best possible hands.
Overview of the common types of documents
Depending on the type of document, mpü specifically adjusts and implements the translation taking into account quality and time management.
mpü offers translation for all types of content and documents and for all areas within your company organisation:
In more than 150 languages for medical translations
mpü translates into and out of all languages and in every required language combination.
No matter what language combination and which subject area you are looking for, mpü has the resources and the experts.
We’d be happy to advise you on the best language solution for your project.









